
Introduction
Dalbavancin is a second generation, semi-synthetic lipoglycopeptide antibiotic that is active against gram-positive pathogens with a MIC90for Staphylococcus aureus of 0.06 μg/mL. Dalbavancin possesses more potent in vitro bactericidal activity than vancomycin or teicoplanin against many resistant Gram-positive organisms such as MRSA. Dalbavanacin displays prolonged terminal half-life in adults.Compared to existing glycopeptides anti-MRSA(Methicillin-Resistant Staphylococcus Aureus) antibiotics, it has better antibiotic strength and, because it only needs to be taken once a week, it will be easier for both patients and medical care providers. It also offers stable absorption and metabolism for patients with light to moderate kidney and liver dysfunction. We know that its interactions with other medications are extremely small, and it is proving to be very safe in clinical trials.
API 99%Min Dalbavancin CAS 171500-79-1
| Product Name: | Dalbavancin |
| Synonyms: | Antibotic Dalbavancin;MDL 63397;A-A-1, Dalbavancin Ao, BI 397, MDL 63397;Dalbavancin(BI 397, MDL 63397);5,31-Dichloro-38-de(methoxycarbonyl)-7-demethyl-19-deoxy-56-O-[2-deoxy-2-[(10-methyl-1-oxoundecyl)amino]-β-D-glucopyranuronosyl]-38-[[[3-(dimethylamino)propyl]amino]carbonyl]-42-O-α-D-mannopyranosyl-N15-methyl-Ristomy |
| CAS: | 171500-79-1 |
| MF: | C88H100Cl2N10O28 |
| MW: | 1816.71 |
| Product Categories: | API |
| Mol File: | 171500-79-1.mol |
| Molecular Structure: | 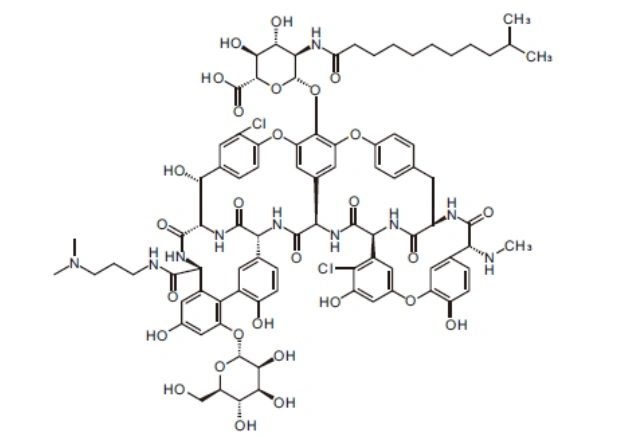 |
Dalbavancin is a new lipoglycopeptide that is active against Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus.
Chemical Properties
| Density | 1.59 |
| Storage temp. | -20°C |
| Form | Powder |
| Color | White to beige |
Uses
Dalbavancin is a semi-synthetic glycopeptide prepared from A40926 by introducing a positively charged lipophilic moiety in a previously unexplored region of the natural glycopeptide. This modification provides a longer in vivo half life, and improved in vitro activity against a variety of Gram positive and multi-drug resistant isolates such as MRSA and MRSE.
Packing & Shipment



Quality Control of Health Sources
1) A system in accordance with GMP standard, supervision on whole production process.
2) Health Sources is equipped with advanced detecting device, such as AFS, GC, HPLC, UV etc, supply the
Detailed Certificate of Analysis.
3) Procurement-Strictly on selecting raw materials.
4) Production-Strictly according to the standard specifications, conform to USP, EP, BP, CP, AJI, FCC etc
Pharmaceutical and/or food standards.
5) Warehousing and storage:Clear and dry condition with suitable temperature.
6) Transportation - Audit and supervise the logistics environment to ensure storage safety.
7) Keep samples for all batches goods to be traceable in case of quality discrepance.
Certification


For small order,you can pay by T/T or Western Union ,nomal order by T/T to our company account.
Q2:Can you give me a discount price?
Surely,It depend on your quantity.
Q3:How can I get a sample?
Free samples is available,but freight charges will be at your account and the charges will be return to you or deduct from your order in the future.
Q4:How to confirm the Product Quality before placing orders?
You can get free samples for some products,you only need to pay the shipping cost or arrange a courier to us and take the samples.You can send us your product specifications and requests,we will manufacture the products according to your requests.
Q5:How do you treat quality complaint?
First of all, our quality control will reduce the quality problem to near zero.If there is a real quality problem caused by us, we will send you free goods for replacement or refund you